- 84286 44000
- 86828 93000 ,95000 ,96000
- admin@linlax.in
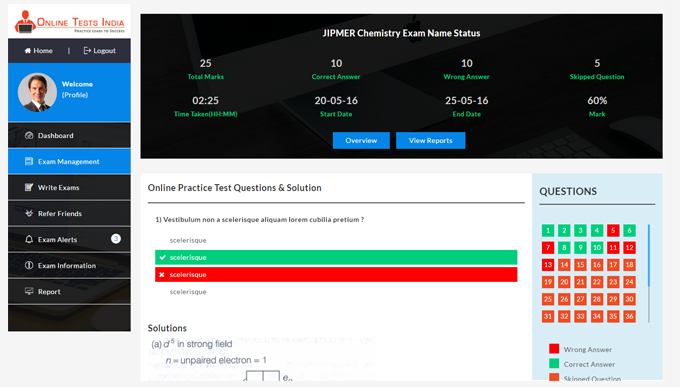
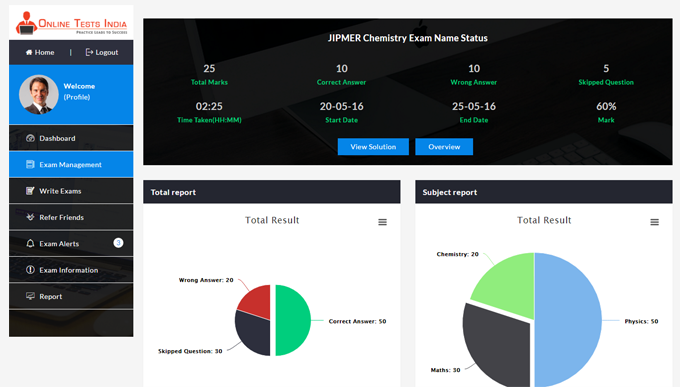
Online Tests India provides a detailed analysis report and also provides in-depth report generation, Instant pie chart creation, improved subject wise grades, track detailed score reports.
Online Tests India provides a detailed analysis report and also provides in-depth report generation, Instant pie chart creation, improved subject wise grades, track detailed score reports.
Online Test India offers a wide range of practice mock tests. Users can be easily write tests and get instant results. Test results were compared with local, regional and national level students.
Question Banks readily available for 12 th standard , Bank Po, SBI-PO , IBPS, RBI EXAM, MBA, MAT, CAT, IIFT, IGNOU, SSC, Hotel Management, Police Exams, Railway Recruitment Board Exams, Campus Recruitment Testsall group exam packs with different question papers
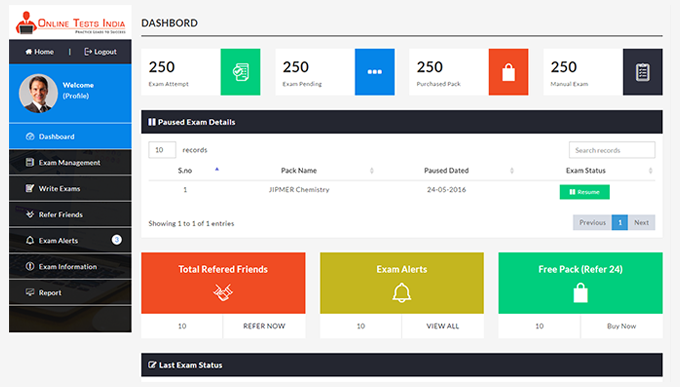
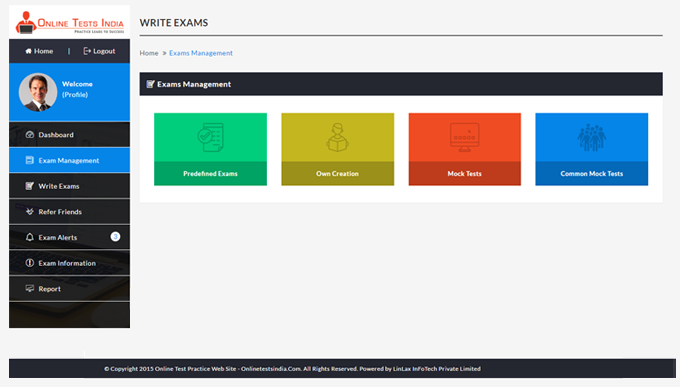
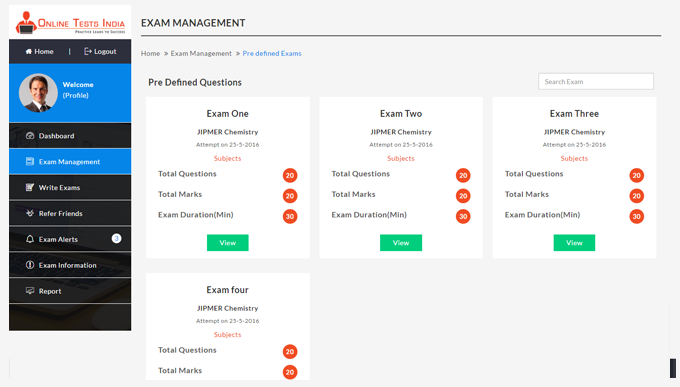
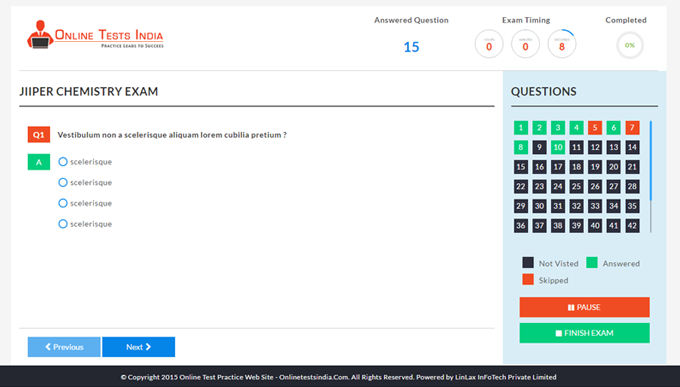
You can access this anywhere in the country, resume option is available for the users.
The workflows which define the working of the software are very easier and simplified.
More than 5,00,000 questions & answer, which included previous year asked questions.
All test results are evaluated instantly and more accurately.