NTSE SAT Chemistry - Classification of Elements
Exam Duration: 45 Mins Total Questions : 30
Which one of the following elements exhibits maximum number of valence electrons?
- (a)
Na
- (b)
Al
- (c)
Si
- (d)
P
Which of the following gives the correct increasing order of the atomic radii of 0, F and N?
- (a)
O, F, N
- (b)
N, F, O
- (c)
O, N, F
- (d)
F, O, N
Which among the following elements has the largest atomic radii?
- (a)
Na
- (b)
Mg
- (c)
K
- (d)
Ca
Which of the following elements does not lose an electron easily?
- (a)
Na
- (b)
F
- (c)
Mg
- (d)
Al
Which of the following are the characteristics of isotopes of an element?
I. Isotopes of an element have same atomic masses.
II. Isotopes of an element have same atomic number.
III. Isotopes of an element show same physical properties.
IV. Isotopes of an element show same chemical properties.
- (a)
(i), (iii) and (iv)
- (b)
(ii), (iii) and (iv)
- (c)
(ii) and (iii)
- (d)
(ii) and (iv)
Arrange the following elements in the order of their decreasing metallic character: Na, Si, CI, Mg, AI
- (a)
CI > Si > AI> Mg > Na
- (b)
Na > Mg > AI > Si > CI
- (c)
Na > AI > Mg > CI > Si
- (d)
AI > Na > Si > Ca > Mg
Arrange the following elements in the order of their increasing non-metallic character: Li, O, C, Be, F
- (a)
F < O< C < Be < Li
- (b)
Li < Be < C < 0< F
- (c)
F < O< C < Be < Li
- (d)
F < O< Be < C < Li
Which of the following elements will form an acidic oxide?
- (a)
An element with atomic number 7
- (b)
An element with atomic number 3
- (c)
An element with atomic number 12
- (d)
An element with atomic number 19
The element with atomic number 14 is hard and forms acidic oxide and a covalent halide. To which of the following categories does the element be?
- (a)
Metal
- (b)
Metalloid
- (c)
Non-metal
- (d)
Left-hand side element
Which one of the following depicts the correct representation of atomic radius(r) of an atom?
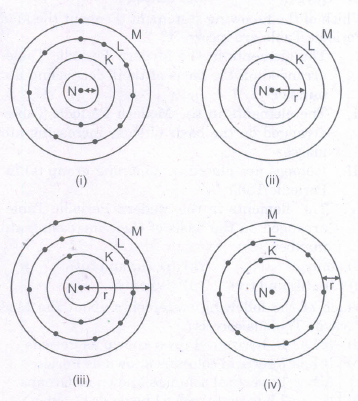
- (a)
(i) and (ii)
- (b)
(ii) and (iii)
- (c)
(iii) and (iv)
- (d)
(i) and (iv)
On moving from left to right in a period in the periodic table, the size of the atom:
- (a)
increase
- (b)
decrease
- (c)
does not change appreciably
- (d)
first decreases and then increases
Which of the following set of elements is written in order of their increasing metallic character?
- (a)
Be, Mg, Ca
- (b)
Na, Li, K
- (c)
Mg, Al, Si
- (d)
C, O, N
In Mendeleev's Periodic Table, gaps were left for the elements to be discovered later. Which of the following elements found a place in the periodic table later:
- (a)
Germanium
- (b)
Chlorine
- (c)
Oxygen
- (d)
Silicon
The elements A, B, C, D and E have atomic number 9, 11, 17, 12 and 13 respectively. Which pair of elements belongs to the same group?
- (a)
A and B
- (b)
B and D
- (c)
A and C
- (d)
D and E
The property of an element in the periodic table depends on its, _____________
- (a)
atomic size
- (b)
atomic mass
- (c)
electronic configuration
- (d)
number of protons
The number of electrons in the valence shell is equal to its _____________
- (a)
atomic mass
- (b)
group number
- (c)
period number
- (d)
atomic number
The non-metallic element present in the third period other than sulphur and chlorine is __________
- (a)
oxygen
- (b)
fluorine
- (c)
nitrogen
- (d)
phosphorus
At the end of each period, the valence shell is _____________
- (a)
incomplete
- (b)
half filled
- (c)
singly occupied
- (d)
completely filled
The family of elements having seven electrons in the outermost shell is ____________
- (a)
alkali metals
- (b)
alkaline earth metals
- (c)
halogens
- (d)
noble gases
Which of the following factors does not affect the metallic character of an element?
- (a)
Atomic size
- (b)
Ionisation potential
- (c)
Electro negativity
- (d)
Atomic radius
The modern periodic table is given by ____________
- (a)
Mendeleev
- (b)
Einstein
- (c)
Bohr
- (d)
Mosley
Elements belonging to groups 1 to 17 are called _____________
- (a)
noble gases
- (b)
normal elements
- (c)
transition elements
- (d)
inner transition elements
A liquid non-metal is ____________
- (a)
phosphorous
- (b)
mercury
- (c)
bromine
- (d)
nitrogen
The first alkali metal is ____________
- (a)
hydrogen
- (b)
lithium
- (c)
sodium
- (d)
francium
A purple coloured solid halogen is ___________
- (a)
chlorine
- (b)
bromine
- (c)
iodine
- (d)
astatine
The family of elements to which calcium belongs is ____________
- (a)
alkali metals
- (b)
alkaline earth metals
- (c)
halogens
- (d)
noble gases
The valency of chlorine with respect to oxygen is _____________
- (a)
1
- (b)
3
- (c)
5
- (d)
7
Six elements A, B, C, D, E and F have the following atomic numbers (A = 12;B = 17, C = 18,D = 7, E = 9 and F = 11). Among these elements, the element, which belongs to the 3rd period and has the highest ionisation potential, is __________ .
- (a)
A
- (b)
B
- (c)
C
- (d)
F
The element, which has zero electron affinity in the 3rd period is _____________.
- (a)
Al
- (b)
P
- (c)
Ar
- (d)
S
The statement that is not true about electron affinity is:
- (a)
It causes energy to be released
- (b)
It causes energy to be absorbed
- (c)
It involves formation of an anion