SAT Physics - Heat
Exam Duration: 45 Mins Total Questions : 30
When the temperature increases the density of a substance
- (a)
decreases
- (b)
increases
- (c)
first increase then decreases
- (d)
first decrease then increases
Two spheres of the same size are made of the same metaI, but one is hollow and the other is solid. When they are heated to the same temperature
- (a)
solid sphere will expand more
- (b)
hollow sphere will expand more
- (c)
only the solid sphere will expand
- (d)
both spheres will expand almost equally
The rate of evaporation of water when it is placed under partial vacuum without changing the temperature will
- (a)
drop to zero
- (b)
decrease
- (c)
increase
- (d)
remain unaffected
When water is heated from 00C, its volume
- (a)
remains the same
- (b)
decrease till 40C
- (c)
increases
- (d)
first increases then decreases
When 1 g of water at 100°C gets converted into steam, at the same temperature the change in volume
- (a)
1cc
- (b)
1000 cc
- (c)
1500 cc
- (d)
1670 cc
In a car, radiator the fan are used to cool the engine. The modes of heat transfer involved are
- (a)
conduction and convection
- (b)
conduction and radiation
- (c)
radiation and convection
- (d)
conduction, convection and radiation
The variation in temperature with time when some wax cools form the liquid phase to the solid phase. The point in which at liquid as well as solid phase is
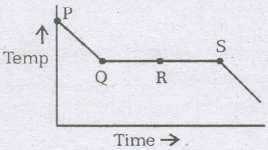
- (a)
P
- (b)
Q
- (c)
R
- (d)
S
Steam produces more burning than water at 1000C because
- (a)
water has high specific heat capacity
- (b)
latent heat of steam
- (c)
steam has high specific heat capacity
- (d)
the temperature of steam is much higher than of water
The amount of heat required to raise the temperature of 100 g of copper from 20°Cto 70°C [specificheat capacity of c~per 390 J kg-1°C-1]
- (a)
1950J
- (b)
3900J
- (c)
1650J
- (d)
780J
1300J of heat is supplied to raise the temperature of 0.5 kg of lead from 20°C to 40°C. e specific heat capacity of lead is
- (a)
700 J kg-1°G-1
- (b)
130 J kg-1°G-1
- (c)
260 J kg-1°G-1
- (d)
0.130 J kg-1°G-1
When 50 cal of heat are supplied to 25 g of water, the rise in temperature is
- (a)
750C
- (b)
20C
- (c)
1250C
- (d)
150C
80 cal of heat is absorbed when
- (a)
1 g of ice melts at 100°C
- (b)
10 g of ice melts at 100°C
- (c)
1 g of ice melts at OOC
- (d)
100g of ice melts at 00C
A temperature difference of 33°C on the kelvin scale is
- (a)
316 K
- (b)
243 K
- (c)
zero
- (d)
33K
At what temperature do the Fahrenheit and celsius scales give the same reading
- (a)
0°
- (b)
273°
- (c)
574°
- (d)
-400
Which of the following metal is used In thermometers?
- (a)
Copper
- (b)
Mercury
- (c)
Aluminium
- (d)
Iron
Which metal is found in liquid state at room temperature?
- (a)
Fe
- (b)
Zn
- (c)
Hg
- (d)
Al
New technologies have been developed to provide thermal energy, without scorching your body. One of these has micro sensors that work like invisible thermostats, that measure the temperature of different parts of your body and generate thermal energy accordingly. This technology is
- (a)
Still in the development stage
- (b)
Found only in research labs
- (c)
An electric blanket
- (d)
Thermal underwear
A technology that has replaced boiling water over an open campfire gives us a warning when the water is boiled. This technology is
- (a)
A micro-sensing digital boiler
- (b)
A solar powered water heater
- (c)
An electric kettle
- (d)
A hot water heater
Absolute zero is a temperature on the Kelvin scale.Although no one has ever been able to cool anything down to absolute zero, scientists know that it is
- (a)
137.15 K
- (b)
237.15 K
- (c)
173.15 K
- (d)
273.15 K
A material, which is affected by changes in some features of the environment, such as temperature is called a
- (a)
Circuit
- (b)
Sensor
- (c)
Signal
- (d)
Responder
Recording thermometers are called thermo-graphs.The 'temperature writer' uses a rotating drum to record changes in temperature. Tiny movements of this device can make large movements on the recording instrument.The device which makes these tiny movements is the:
- (a)
Lever
- (b)
Pen
- (c)
Bimetallic strip
- (d)
Rotating drum
Another important idea about temperature and the particle theory is that the motion of particles increases when the temperature increases. Which of statements below is correct?
- (a)
As the motion of particles decreases, the temperature remains the same
- (b)
As the temperature decreases, the motion of the particles also increases
- (c)
As the motion of the particles decreases, the temperature decreases
- (d)
As the temperature increases, the motion of the particles decreases
Solids made of different metals were all heated to 100°C to determine how their volume and length would be affected. Which statement describes the most likely outcome of this experiment?
- (a)
All the volumes changed the same amount and the lengths remained constant
- (b)
All the volumes changed, but each substance was of the same length
- (c)
Only some of the volumes changed with their length being increased
- (d)
All the volumes changed and so did their lengths
When a substance undergoes a change of state, energy is involved. Which change of state involves a release of energy?
- (a)
Melting
- (b)
Sublimation
- (c)
Evaporation
- (d)
Fusion
During a phase change, the temperature remains the same, so the particles have:
- (a)
Less average energy
- (b)
More average energy
- (c)
The same average energy
- (d)
A faster speed
Programmable thermostats can be used while the occupant of the home is asleep or away. These devices
- (a)
Adjust the temperature
- (b)
Increase the temperature
- (c)
Decrease the temperature
- (d)
Reduce the humidity
An ENERGUIDE label is found on most household electrical appliances and tells the consumer how much electricity is:
- (a)
Needed to run the appliance
- (b)
Used while running the appliance
- (c)
Wasted by the appliance
- (d)
Generated while running the appliance
______ is the primary source of heat on Earth
- (a)
Sun
- (b)
Fire
- (c)
Moon
- (d)
Fossil fuels
In olden times ________ was the only method for producing heat.
- (a)
Friction
- (b)
Coal
- (c)
Oil
- (d)
Gas
A substance in the fused state means a substance in the ______ state.
- (a)
Solid
- (b)
Combined
- (c)
Molten
- (d)
Gaseous